The rule is you use the set of numbers that has the lowest number at the first point of difference. In that molecule there are two valid ways to number the molecule and the sets of numbers are 2,3,3,7,7 and 2,2,6,6,7. In the second set there is a 2 for the second number, whereas it’s 3 for the first set.
ORGANIC CHEMISTRY 1 Lecture 1 Different manners of writing and drawing – ppt download
Example 4.1. 1. The longest straight chain contains 10 carbons, compared with cyclopropane, which only contains 3 carbons. The parent chain in this molecule is decane and cyclopropane is a substituent. The name of this molecule is 3-cyclopropyl-6-methyldecane. Example 4.1. 2.

Source Image: youtube.com
Download Image
02:18 , Jay draws bond line structure for propane. How to draw methane with this shorthand (bond-line structure) method? A single point would look like a bit unclear. So i guess they apply bond-line structure to structures consisting of at least ethane-like structure. Am i right? • ( 12 votes) Upvote Flag dysmnemonic
Source Image: chegg.com
Download Image
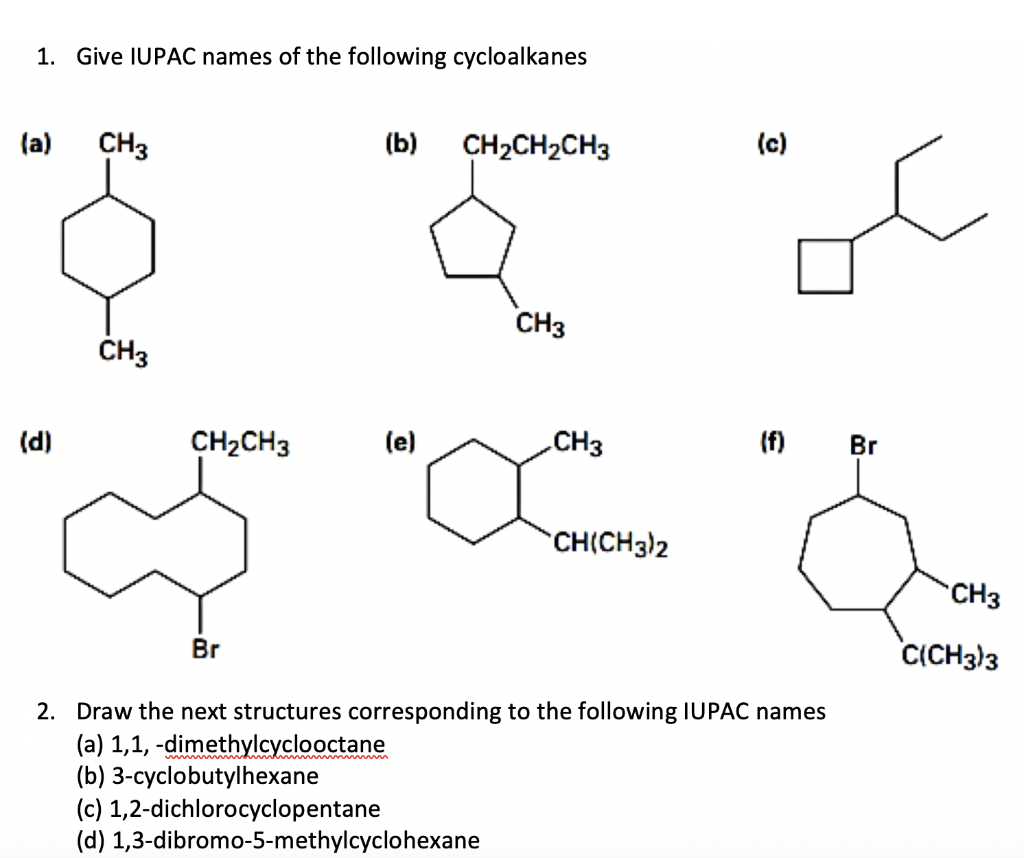
SOLUTION: Examples of Common Names and IUPAC of Important Organic Compounds (IIT JEE NEET MAINS DU HARVARD UNIVERSITY SEU UNIVRSITY) – Studypool Expertverified 100% (3 ratings) Step 1 As per the given question, The objective of the question is to select the correct IUPAC name of the … View the full answer Step 2 Unlock Step 3 Unlock Answer Unlock Previous question Next question Transcribed image text:

Source Image: scribd.com
Download Image
Select The Correct Iupac Name For The Cycloalkane:
Expertverified 100% (3 ratings) Step 1 As per the given question, The objective of the question is to select the correct IUPAC name of the … View the full answer Step 2 Unlock Step 3 Unlock Answer Unlock Previous question Next question Transcribed image text: Science Chemistry Chemistry questions and answers Select the correct IUPAC name for the cycloalkane: The IUPAC name is:2‑bromo‑4‑fluoro‑1‑propylcyclohexane1‑propyl‑2‑bromo‑4‑fluorocyclohexane3‑bromo‑1‑fluoro‑4‑propylcyclohexane1‑bromo‑3‑fluoro‑6‑propylcyclohexane This problem has been solved!
Organic DPT 1-14 | PDF | Carboxylic Acid | Amine
Lesson 1: Naming alkanes Naming alkanes with alkyl groups Correction – 2-propylheptane should never be the name! Common and systematic naming: iso-, sec-, and tert- prefixes Naming alkanes with ethyl groups Alkane with isopropyl group Organic chemistry naming examples 2 Organic chemistry naming examples 3 Naming a cycloalkane SOLUTION: Examples of Common Names and IUPAC of Important Organic Compounds 2 (IIT JEE NEET MAINS DU HARVARD UNIVERSITY SEU UNIVRSITY) – Studypool

Source Image: studypool.com
Download Image
IUPAC From Arihant | PDF | Alkane | Functional Group Lesson 1: Naming alkanes Naming alkanes with alkyl groups Correction – 2-propylheptane should never be the name! Common and systematic naming: iso-, sec-, and tert- prefixes Naming alkanes with ethyl groups Alkane with isopropyl group Organic chemistry naming examples 2 Organic chemistry naming examples 3 Naming a cycloalkane

Source Image: scribd.com
Download Image
ORGANIC CHEMISTRY 1 Lecture 1 Different manners of writing and drawing – ppt download The rule is you use the set of numbers that has the lowest number at the first point of difference. In that molecule there are two valid ways to number the molecule and the sets of numbers are 2,3,3,7,7 and 2,2,6,6,7. In the second set there is a 2 for the second number, whereas it’s 3 for the first set.

Source Image: slideplayer.com
Download Image
SOLUTION: Examples of Common Names and IUPAC of Important Organic Compounds (IIT JEE NEET MAINS DU HARVARD UNIVERSITY SEU UNIVRSITY) – Studypool 02:18 , Jay draws bond line structure for propane. How to draw methane with this shorthand (bond-line structure) method? A single point would look like a bit unclear. So i guess they apply bond-line structure to structures consisting of at least ethane-like structure. Am i right? • ( 12 votes) Upvote Flag dysmnemonic

Source Image: studypool.com
Download Image
SOLUTION: Short summary of iupac nomenclature of organic compounds – Studypool However, the common names do not generally follow the basic IUPAC nomenclature rules. The general formula of the cycloalkanes is CnH2n C n H 2 n where n n is the number of carbons. The naming of cycloalkanes follows a simple set of rules that are built upon the same basic steps in naming alkanes. Cyclic hydrocarbons have the prefix “cyclo-“.

Source Image: studypool.com
Download Image
SOLUTION: IUPAC Nomenclature of Organic Compounds Review Paper – Studypool Expertverified 100% (3 ratings) Step 1 As per the given question, The objective of the question is to select the correct IUPAC name of the … View the full answer Step 2 Unlock Step 3 Unlock Answer Unlock Previous question Next question Transcribed image text:

Source Image: studypool.com
Download Image
Solved] Select the correct IUPAC name for the cycloalkane: . X The IUPAC… | Course Hero Science Chemistry Chemistry questions and answers Select the correct IUPAC name for the cycloalkane: The IUPAC name is:2‑bromo‑4‑fluoro‑1‑propylcyclohexane1‑propyl‑2‑bromo‑4‑fluorocyclohexane3‑bromo‑1‑fluoro‑4‑propylcyclohexane1‑bromo‑3‑fluoro‑6‑propylcyclohexane This problem has been solved!
Source Image: coursehero.com
Download Image
IUPAC From Arihant | PDF | Alkane | Functional Group
Solved] Select the correct IUPAC name for the cycloalkane: . X The IUPAC… | Course Hero Example 4.1. 1. The longest straight chain contains 10 carbons, compared with cyclopropane, which only contains 3 carbons. The parent chain in this molecule is decane and cyclopropane is a substituent. The name of this molecule is 3-cyclopropyl-6-methyldecane. Example 4.1. 2.
SOLUTION: Examples of Common Names and IUPAC of Important Organic Compounds (IIT JEE NEET MAINS DU HARVARD UNIVERSITY SEU UNIVRSITY) – Studypool SOLUTION: IUPAC Nomenclature of Organic Compounds Review Paper – Studypool However, the common names do not generally follow the basic IUPAC nomenclature rules. The general formula of the cycloalkanes is CnH2n C n H 2 n where n n is the number of carbons. The naming of cycloalkanes follows a simple set of rules that are built upon the same basic steps in naming alkanes. Cyclic hydrocarbons have the prefix “cyclo-“.