Xin Liu Kwantlen Polytechnic University via Kwantlen Polytechnic University S N 2 Reaction Mechanism Let’s still take the reaction between CH 3 Br and OH – as the example for S N 2 mechanism. S N 2 mechanism involves two electron pair transfers that occur at the same time, nucleophile attacking (red arrow) and leave group leaving (blue arrow).
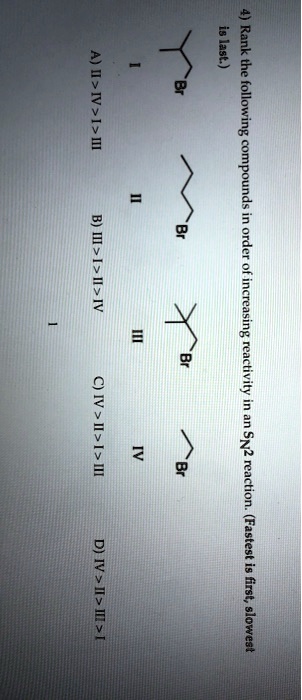
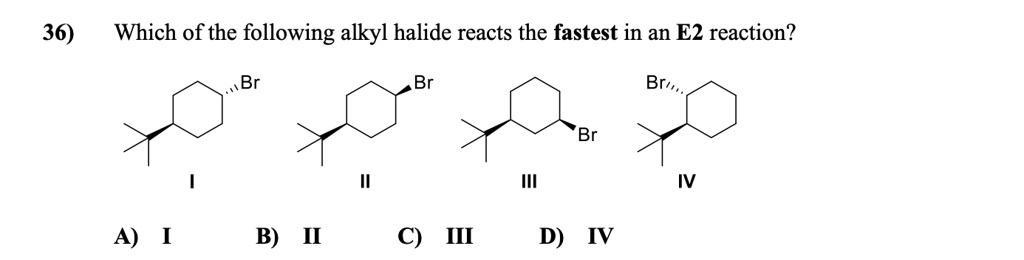
SOLVED: Which of the following alkyl halides reacts the fastest in an E2 reaction? Br Br Brv, , Br A) I B) II C) III D) IV
Solution Verified by Toppr A standard s2 N reaction can be shon a. It is clear that, more the tendancy of x to leave the carbon, mere faster the reaction olll occur. For halogens, learing group order: [I >Br >cl >F nence alkyl group having ‘I’ as leaving group attached sill be fastest. so, option C is correct. Was this answer helpful? 0
 Download Image
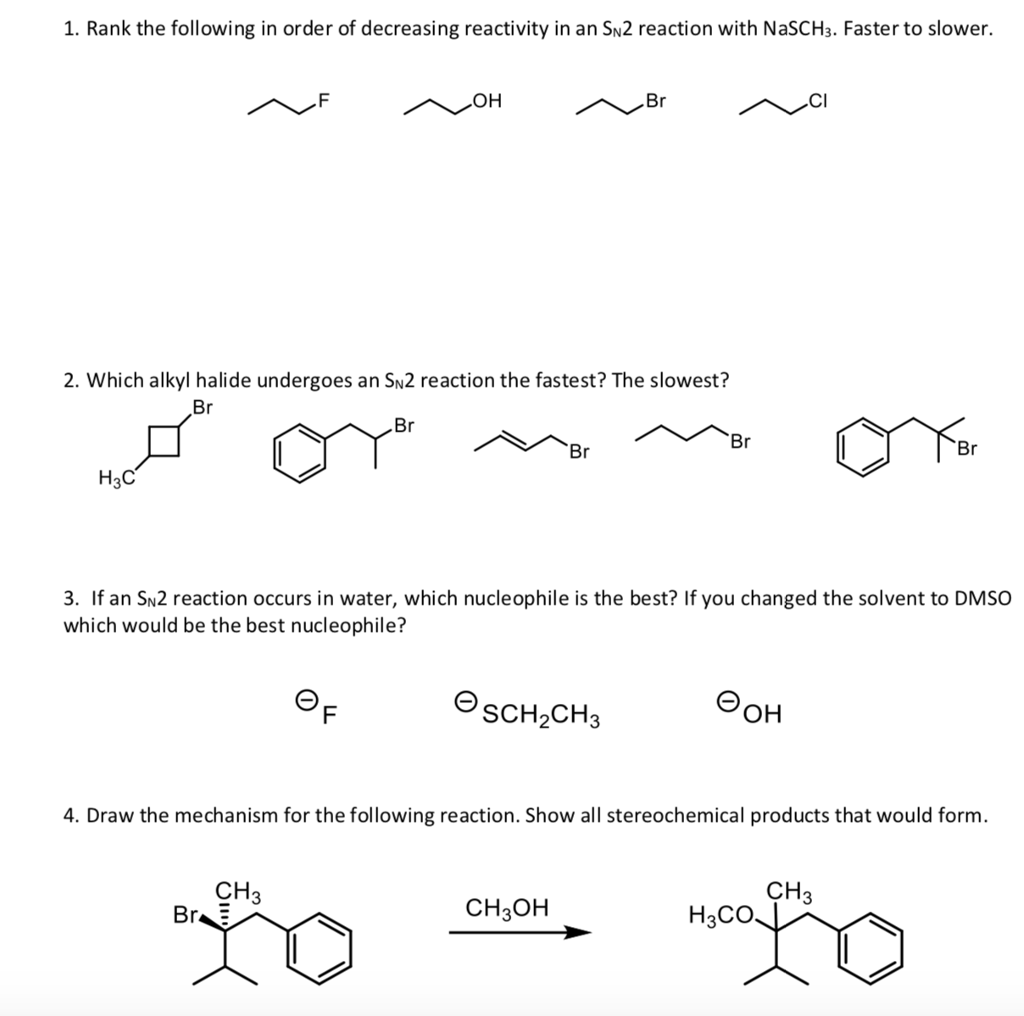
Download Image1. The SN2 Reaction Proceeds With Inversion of Configuration When we start with a molecule with a chiral center, such as (S)-2-bromobutane, this class of reaction results in inversion of stereochemistry. Note how we start with (S)-2-bromobutane and end up with (R)-2-methylbutanenitrile. 2. The Rate Law Of The SN2 Is Second Order Overall
Source Image: chegg.com
Download Image
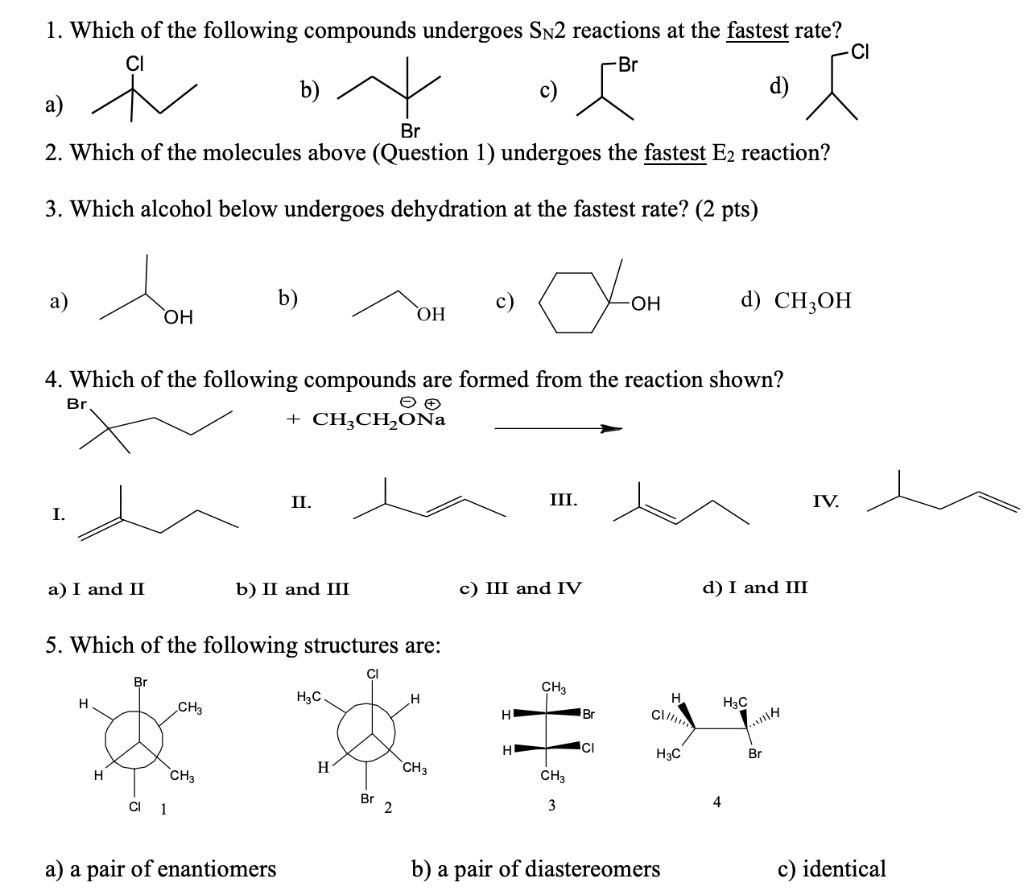
SOLVED: 1. Which of the following compounds undergoes Sn2 reactions at the fastest rate? Cl Br Br 2. Which of the molecules above (Question 1) undergoes the fastest E2 reaction? 3. Which Science > Organic chemistry (Essentials) – Class 12 > Nucleophilic substitution reactions of alkyl halides, alcohols and ethers > SN2 Practice questions on SN2 reactions Consider the reaction given below: Predict the major product in the given reaction. Choose 1 answer: A B C Stuck? Use a hint. Report a problem Do 4 problems
Source Image: numerade.com
Download Image
Which Of The Following Sn2 Reactions Is The Fastest
Science > Organic chemistry (Essentials) – Class 12 > Nucleophilic substitution reactions of alkyl halides, alcohols and ethers > SN2 Practice questions on SN2 reactions Consider the reaction given below: Predict the major product in the given reaction. Choose 1 answer: A B C Stuck? Use a hint. Report a problem Do 4 problems Science Chemistry Chemistry questions and answers Which of the following SN2 reactions is the fastest This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: Which of the following SN2 reactions is the fastest
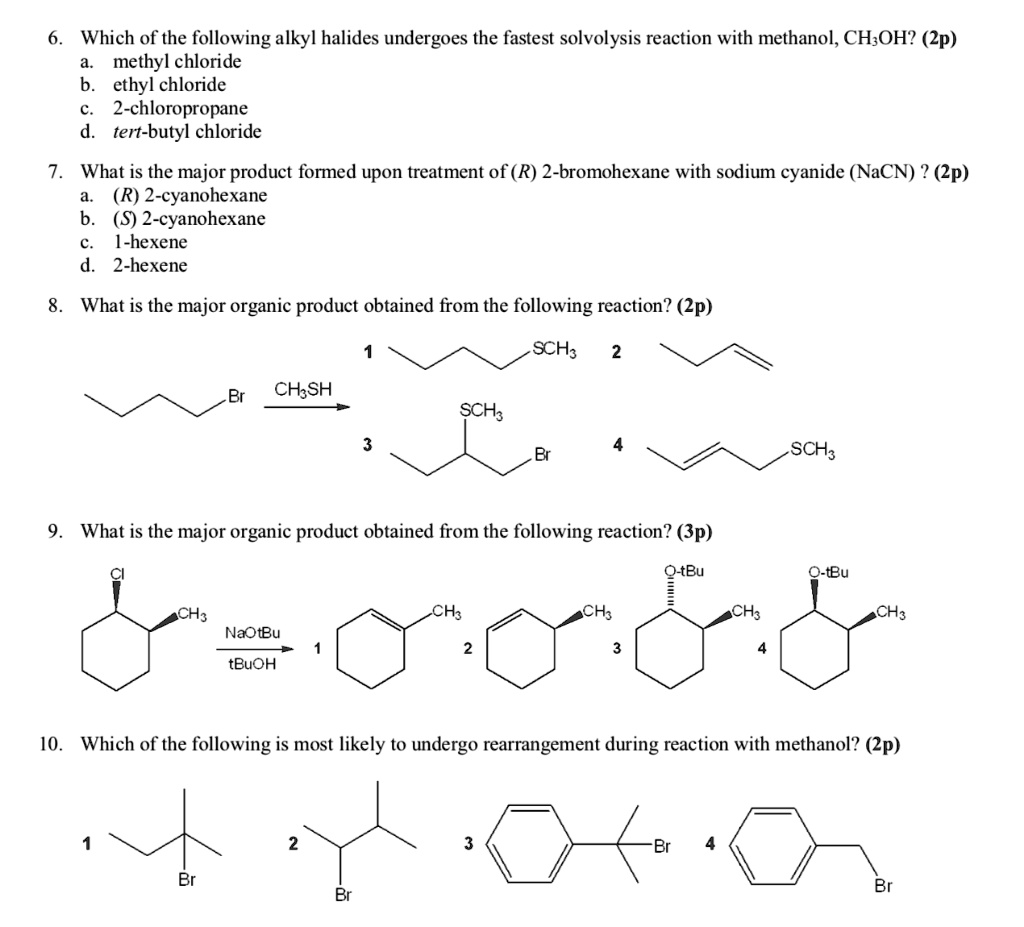
SOLVED: Which of the following alkyl halides undergoes the fastest solvolysis reaction with methanol, CH3OH? (Zp) methyl chloride, ethyl chloride, 2-chloropropane, tert-butyl chloride. What is the major product formed upon treatment of (
The bond-making between the nucleophile and the electrophilic carbon occurs at the same time as the bond-breaking between the electophilic carbon and the halogen. In order of decreasing importance, the factors impacting S N 2 reaction pathways are. 1) structure of the alkyl halide. 2) strength of the nucleophile. 3) stability of the leaving group. Deciding SN1/SN2/E1/E2 (1) – The Substrate – Master Organic Chemistry
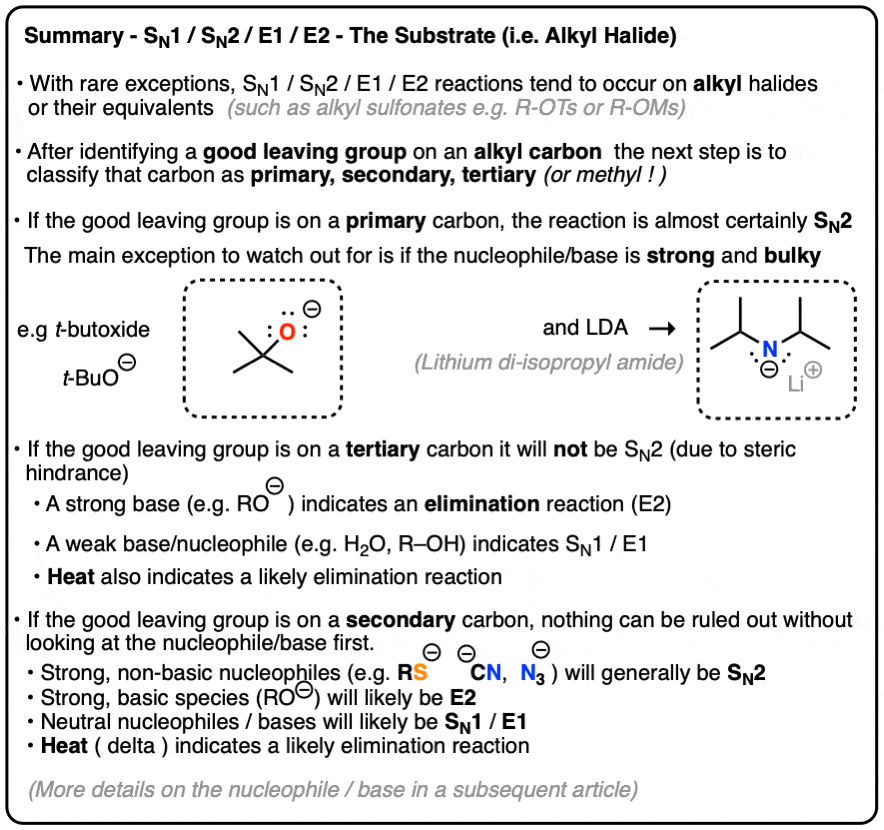
Source Image: masterorganicchemistry.com
Download Image
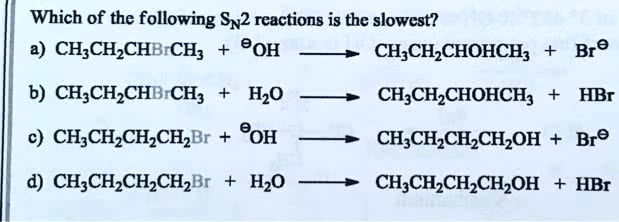
SOLVED: Which of the following SN2 reactions is the slowest? CH3CH2CHOHCHBr® The bond-making between the nucleophile and the electrophilic carbon occurs at the same time as the bond-breaking between the electophilic carbon and the halogen. In order of decreasing importance, the factors impacting S N 2 reaction pathways are. 1) structure of the alkyl halide. 2) strength of the nucleophile. 3) stability of the leaving group.
Source Image: numerade.com
Download Image
SOLVED: Which of the following alkyl halides reacts the fastest in an E2 reaction? Br Br Brv, , Br A) I B) II C) III D) IV Xin Liu Kwantlen Polytechnic University via Kwantlen Polytechnic University S N 2 Reaction Mechanism Let’s still take the reaction between CH 3 Br and OH – as the example for S N 2 mechanism. S N 2 mechanism involves two electron pair transfers that occur at the same time, nucleophile attacking (red arrow) and leave group leaving (blue arrow).
Source Image: numerade.com
Download Image
SOLVED: 1. Which of the following compounds undergoes Sn2 reactions at the fastest rate? Cl Br Br 2. Which of the molecules above (Question 1) undergoes the fastest E2 reaction? 3. Which 1. The SN2 Reaction Proceeds With Inversion of Configuration When we start with a molecule with a chiral center, such as (S)-2-bromobutane, this class of reaction results in inversion of stereochemistry. Note how we start with (S)-2-bromobutane and end up with (R)-2-methylbutanenitrile. 2. The Rate Law Of The SN2 Is Second Order Overall
Source Image: numerade.com
Download Image
arrange the following compounds in order of sn2 reaction:1)ch3(ch2)3ch2Br; (2) (ch3)2chch2ch2Br ;3) ch3ch2ch(ch3)ch2Br;4) ch3(ch3)2cch2B S N2 reaction is bimolecular reaction which takes place by the formation of T.S. Velocity of the reaction depends on the concentration of the substrate as well as the nucleophile. The reaction is favoured by strong Nu ⊖ and in the presence of polar aprotic solvent, optically active halides give Walden inversion by S N2 mechanism; The presence
Source Image: byjus.com
Download Image
Which of the following SN2 reactions would be the fastest? [Image src=’reaction915376329492630519.jpg’ alt=’reaction’ caption=”] | Homework.Study.com Science > Organic chemistry (Essentials) – Class 12 > Nucleophilic substitution reactions of alkyl halides, alcohols and ethers > SN2 Practice questions on SN2 reactions Consider the reaction given below: Predict the major product in the given reaction. Choose 1 answer: A B C Stuck? Use a hint. Report a problem Do 4 problems
![Which of the following SN2 reactions would be the fastest? [Image src='reaction915376329492630519.jpg' alt='reaction' caption=''] | Homework.Study.com](https://homework.study.com/cimages/multimages/16/reaction915376329492630519.jpg)
Source Image: homework.study.com
Download Image
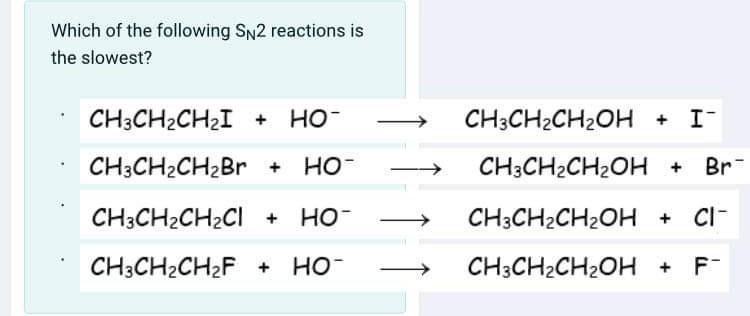
Solved Which of the following Sn2 reactions is the slowest? | Chegg.com Science Chemistry Chemistry questions and answers Which of the following SN2 reactions is the fastest This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: Which of the following SN2 reactions is the fastest
Source Image: chegg.com
Download Image
SOLVED: Which of the following SN2 reactions is the slowest? CH3CH2CHOHCHBr®
Solved Which of the following Sn2 reactions is the slowest? | Chegg.com Solution Verified by Toppr A standard s2 N reaction can be shon a. It is clear that, more the tendancy of x to leave the carbon, mere faster the reaction olll occur. For halogens, learing group order: [I >Br >cl >F nence alkyl group having ‘I’ as leaving group attached sill be fastest. so, option C is correct. Was this answer helpful? 0
SOLVED: 1. Which of the following compounds undergoes Sn2 reactions at the fastest rate? Cl Br Br 2. Which of the molecules above (Question 1) undergoes the fastest E2 reaction? 3. Which Which of the following SN2 reactions would be the fastest? [Image src=’reaction915376329492630519.jpg’ alt=’reaction’ caption=”] | Homework.Study.com S N2 reaction is bimolecular reaction which takes place by the formation of T.S. Velocity of the reaction depends on the concentration of the substrate as well as the nucleophile. The reaction is favoured by strong Nu ⊖ and in the presence of polar aprotic solvent, optically active halides give Walden inversion by S N2 mechanism; The presence